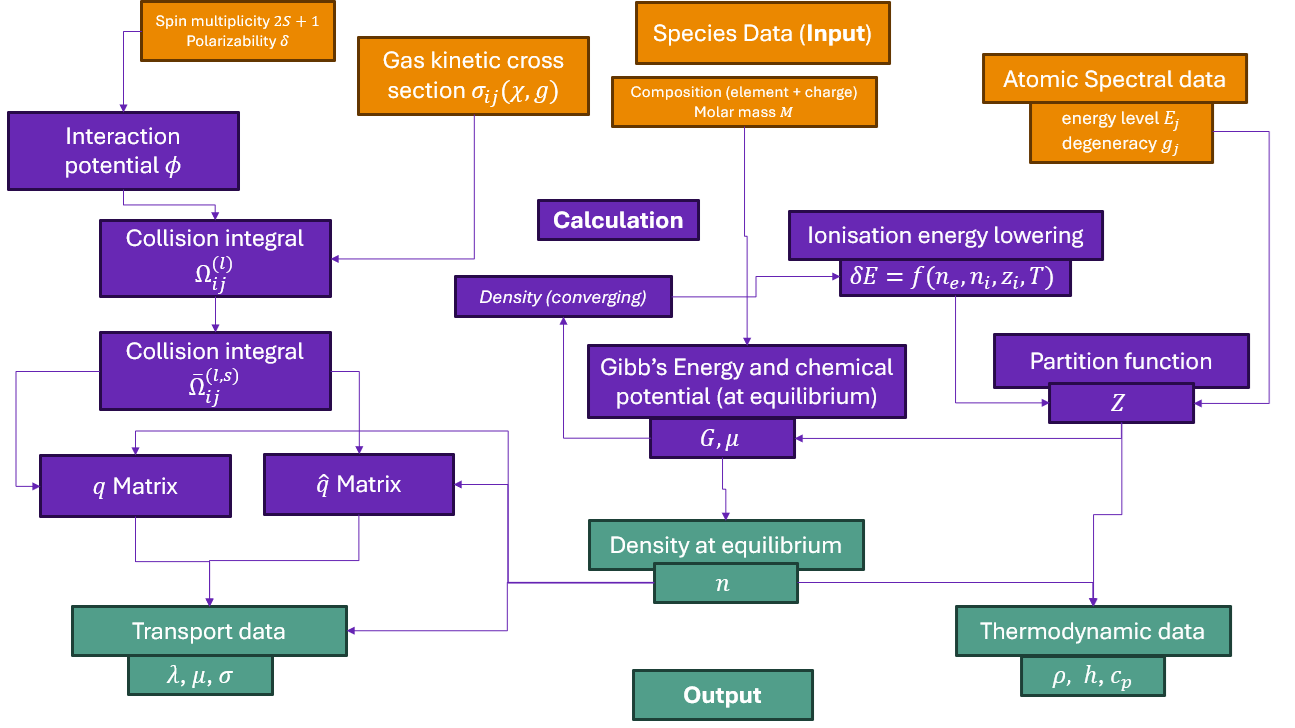
Tutorial 08: Calculating equilibrium compositions of an SiO-CO plasma.#
Calculations of plasmas arising from metallurgical processes are typically a lot more complicated than single-element systems. For example the plasma in an electric arc running above an oxide slag or sulphide matte in a smelting furnace may have multiple metallic elements and their molecules present in different proportions, together with carbon, oxygen, sulphur, and trace impurities. To illustrate this let’s consider the case of a carbon monoxide & silicon monoxide plasma, such as one might obtain when heating pure \(SiO_2\) in a reducing environment with carbon present.
A small text file in the JSON format must be generated by the user to specify the system. In this case we’ll assume the presence of as many possible species obtained by combining silicon, carbon, and oxygen as we have data for, but it’s important to be aware that such a “brute-force” approach may not always be the most expedient as the number of species rises; the expertise of the user in judging which species are of dominant importance in a particular plasma mixture becomes valuable in such cases.
We’ll go ahead and assume that all of \(O_2, O_2^+, O, O^+, O^{2+}, SiO, SiO^+, Si, Si^+, Si^{2+}, CO, CO^+, C, C^+, C^{2+}\) are present, and let’s also assume we are interested in the case of a plasma formed from a 50/50 mixture (by mole) of CO and SiO. This composition constraint has the effect of fixing the elemental make-up of the plasma using mole fractions of the two diatomic species CO and SiO; what this really amounts to internally is telling minplascalc that “at all times during the calculation of the composition, you should have 1 atom of carbon and 1 atom of silicon for every 2 atoms of oxygen regardless of their proportionation between different species”, thereby preserving the element balance for C, Si, and O.
In order to calculate the composition of the plasma at various temperatures using these species, execute the following code snippets in order. The text in between indicates what each part of the code is doing.
Import the required libraries.#
We start by importing the modules we need:
matplotlib for drawing graphs,
numpy for array functions,
and of course minplascalc.
import matplotlib.pyplot as plt
import numpy as np
import minplascalc as mpc
Create mixture object for the species we’re interested in.#
Next, we create a minplascalc LTE mixture object, again using the helper function in minplascalc which creates the object directly from a list of the species names.
species = [
"O2",
"O2+",
"O",
"O+",
"O++",
"CO",
"CO+",
"C",
"C+",
"C++",
"SiO",
"SiO+",
"Si",
"Si+",
"Si++",
]
x0 = [0, 0, 0, 0, 0, 0.5, 0, 0, 0, 0, 0.5, 0, 0, 0, 0]
sico_mixture = mpc.mixture.lte_from_names(species, x0, 1000, 101325)
Set a range of temperatures to calculate the equilibrium compositions at.#
Next, set a range of temperatures to calculate the equilibrium compositions at - in this case we’re going from 1000 to 25000 K in 100 K steps. Also initialise a list to store the composition result at each temperature
temperatures = np.linspace(1000, 25000, 100)
species_names = [sp.name for sp in sico_mixture.species]
ni_list = []
Perform the composition calculations.#
Now we’re ready to actually perform the composition calculations. We loop over all the temperatures, setting the LTE object’s temperature attribute to the appropriate value, and calculating the composition using the object’s calculate_composition() function.
Note that execution of this calculation is fairly compute intensive (the more species present the more intensive it becomes) and the following code snippet may take a few tens of seconds to complete.
for T in temperatures:
sico_mixture.T = T
ni_list.append(sico_mixture.calculate_composition())
ni = np.array(ni_list).transpose()
Plot the results.#
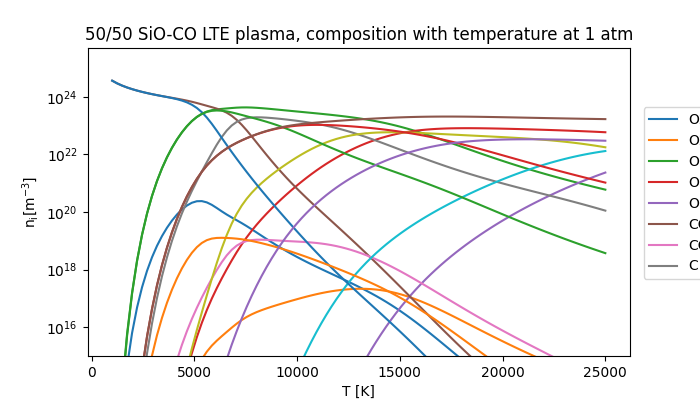
Now we can go ahead and visualise the results by plotting the plasma composition against temperature, to see how it varies.
fig, ax = plt.subplots(1, 1, figsize=(7, 4))
ax.set_title("50/50 SiO-CO LTE plasma, composition with temperature at 1 atm")
ax.set_xlabel("T [K]")
ax.set_ylabel(r"$\mathregular{n_i [m^{-3}]}$")
ax.set_ylim(1e15, 5e25)
for spn, sn in zip(ni, species_names):
ax.semilogy(temperatures, spn, label=sn)
ax.legend(loc=(1.025, 0.25), ncols=2)
<matplotlib.legend.Legend object at 0x7ff837996990>
Reducing the number of species to plot.#
Whew, what a mess! It’s hard to see what’s going on in this complex plasma, so let’s pull out just the silicon species and the pure oxygen species.
silicon_species = ["SiO", "SiO+", "Si", "Si+", "Si++"]
silicon_species_names = []
silicon_ni = []
for spn, sn in zip(ni, species_names):
if sn in silicon_species:
silicon_species_names.append(sn)
silicon_ni.append(spn)
oxygen_species = ["O2", "O2+", "O", "O+", "O++"]
oxygen_species_names = []
oxygen_ni = []
for spn, sn in zip(ni, species_names):
if sn in oxygen_species:
oxygen_species_names.append(sn)
oxygen_ni.append(spn)
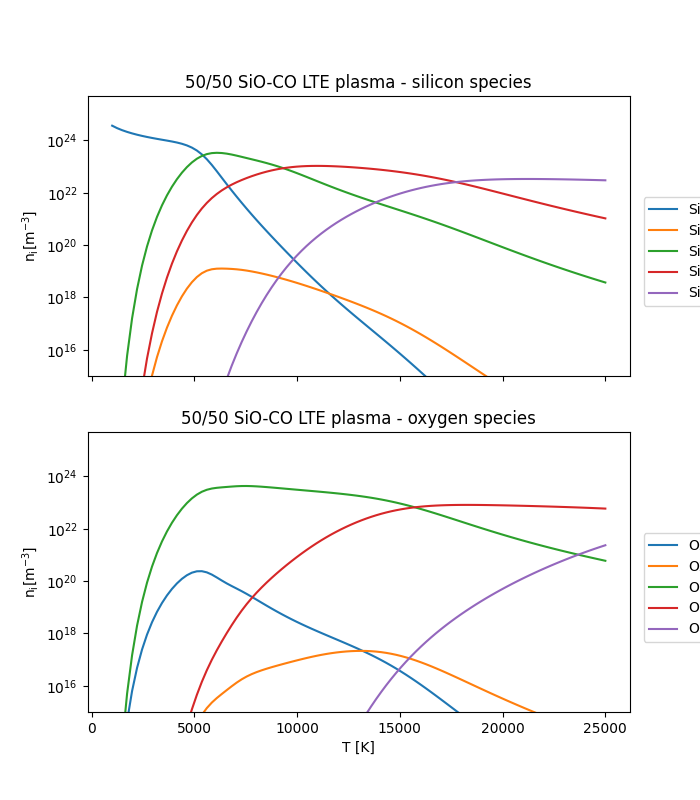
Plotting those side by side we can start to see some more useful information.
fig, axs = plt.subplots(2, 1, figsize=(7, 8), sharex=True)
ax = axs[0]
ax.set_title("50/50 SiO-CO LTE plasma - silicon species")
ax.set_ylabel(r"$\mathregular{n_i [m^{-3}]}$")
ax.set_ylim(1e15, 5e25)
for spn, sn in zip(silicon_ni, silicon_species_names):
ax.semilogy(temperatures, spn, label=sn)
ax.legend(loc=(1.025, 0.25))
ax = axs[1]
ax.set_title("50/50 SiO-CO LTE plasma - oxygen species")
ax.set_xlabel("T [K]")
ax.set_ylabel(r"$\mathregular{n_i [m^{-3}]}$")
ax.set_ylim(1e15, 5e25)
for spn, sn in zip(oxygen_ni, oxygen_species_names):
ax.semilogy(temperatures, spn, label=sn)
ax.legend(loc=(1.025, 0.25))
<matplotlib.legend.Legend object at 0x7ff83788a2d0>
Conclusion#
That’s a bit better. Now we can see that the general behaviour of SiO in this mixture is quite similar to that of \(O_2\) (see Tutorial 05: Calculating equilibrium compositions for an O_2 plasma.), # noqa: E501 with the difference that Si species are easier to ionise - this leads to higher concentrations of charged ions, particularly at lower temperatures.
It also suggests that at higher temperatures, our calculation should probably be including \(Si^{3+}\) as well. Pure oxygen species are largely absent from the plasma at low temperatures as one would expect (this is after all a CO-SiO plasma with no stoichiometrically free oxygen), but as dissociation and ionisation of the other species proceeds their concentrations rise and start to contribute significantly to the plasma composition.
Total running time of the script: (0 minutes 8.231 seconds)